Uncategorized Friday, 2024/07/19
Ultraviolet (UV) radiation induces DNA damage and activates the DNA damage response (DDR) pathway, thereby blocking the cell cycle and allowing time for DNA repair. When DNA damage becomes severe and difficult to repair, it activates cell apoptosis. The study of UV radiation-induced DNA damage has long been well-known, but there is relatively little mention of how UV can also induce RNA damage. Research has found that UV can generate pyrimidine dimers and other light products through photochemical reactions, which can induce RNA damage within the transcriptome. UV damage can also lead to translation defects, causing ribosomes to stagnate on damaged codons rich in pyrimidine, leading to ribosome collisions and activating ribotoxic stress response (RSR). RSR will activate downstream ZAK and GCN2 kinases. ZAK kinase activates the MAPK pathway to mediate cell cycle arrest and apoptosis, while GCN2 kinase activation leads to eIF2 α phosphorylation, and inhibition of protein synthesis mediated by integrated stress response (ISR). Although UV-induced RNA damage has been found to activate the RSR and ISR pathways through ribosome collision, these studies have failed to address a key question: how cells integrate these responses to determine their fate.
Rachel Green's research group from Johns Hopkins University School of Medicine in the United States has published a paper titled 'The ribotoxic stress response drives UV-mediated cell death' in Cell. In this study, the author comprehensively analyzed the various nucleic acid damage stress responses that occur in cells after UV irradiation and found that RSR plays a more critical role in UV-dependent programmed cell death than DDR. This discovery breaks through traditional understanding and provides new insights into how cells respond to UV damage.
Our Related Proteins
| Cat. No. | Product Name | Source | Species | Tag |
| ZAK-1510H | Active Recombinant Human ZAK, GST-tagged | Sf9 Insect Cell | Human | GST |
| ZAK-457H | Recombinant Human ZAK, GST-tagged, Active | Sf9 Insect Cell | Human | GST |
| ZAK-224HCL | Recombinant Human ZAK 293 Cell Lysate | HEK293 | Human | N/A |
| EIF2AK4-3747HF | Active Recombinant Full Length Human EIF2AK4 Protein, GST-tagged | Insect (sf21) | Human | GST |
| EIF2AK4-3346H | Recombinant Human EIF2AK4 protein, His-tagged | E.coli | Human | His |
| EIF2AK4-1414H | Recombinant Human EIF2AK4 Protein, His-tagged | E.coli | Human | N-His |
| EIF2AK4-2310HF | Active Recombinant Full Length Human EIF2AK4 Protein, DDK-tagged, Biotinylated | Insect (sf21) | Human | DDK |
| AHSA1-9499H | Recombinant Human AHSA1, GST-tagged | E.coli | Human | GST |
| ATR-1026H | Recombinant Human ATR protein, GST-tagged | Wheat Germ | Human | GST |
| EIF2A-12348H | Recombinant Human EIF2A, GST-tagged | E.coli | Human | GST |
| MTOR-30254TH | Recombinant Human MTOR | Wheat Germ | Human | N/A |
To clarify this issue, the author first studied the graded response of cells to UV intensity. At lower levels of UV radiation, cells mainly stagnate in the G2 phase, with very few dead cells; Higher UV intensity leads to a significant increase in the proportion of apoptotic cells. Accumulation of ribosome collisions can be observed within minutes after low-dose UV irradiation and cleared within a few hours. Analysis of the activation kinetics of the RSR, ISR, and DDR signaling pathways after low-dose UV irradiation revealed that: (1) ZAK kinase, which represents the RSR pathway, is phosphorylated within minutes and reaches its peak at 15 minutes, while p38 and JNK downstream of ZAK are rapidly activated, indicating that the RSR pathway is highly synergistic in early response; (2) EIF2 α in the ISR pathway is phosphorylated early and continuously increases after UV irradiation, indicating that the ISR signaling pathway gradually strengthens in response to UV irradiation; (3) The phosphorylation rate of effector molecules in DDR is slower, exhibiting a different activation mode from RSR and ISR. The author further confirmed this discovery through phosphorylation and proteomic analysis, that is, the sequential activation patterns of different stress responses under UV irradiation, and emphasized the dominant role of ribosome-mediated signal transduction in the early stages.
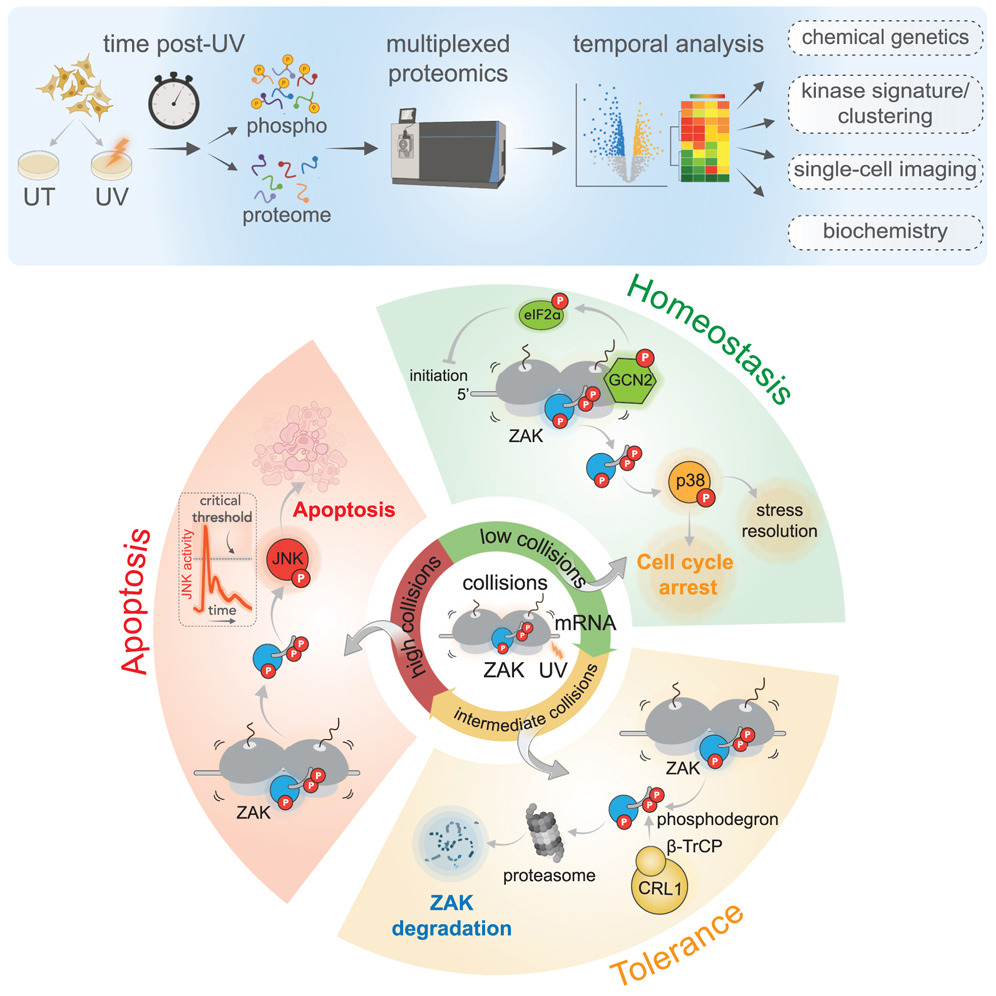
Next, the author conducted a detailed study on the effects of ZAK and GCN2 on the changes in phosphorylated proteome after UV treatment. By knocking out ZAK or GCN2 and treating with specific inhibitors of both kinases, the authors found that ZAK plays a key role in early response, while GCN2 plays an important role in regulating p38 effector kinase.
In addition, the RSR and DDR signaling pathways operate independently during UV response, as treatment with inhibitors of DDR effector kinase ATR revealed no correlation between DDR-specific phosphorylation sites and ZAK kinase-dependent phosphorylation sites. After analyzing the roles of ZAK and ATR in determining cell fate, the authors found that ZAK mainly drives early apoptosis and G2/M checkpoint activation in UV response, while ATR plays a key role in cell proliferation and DNA repair. Correspondingly, GCN2 inhibits translation initiation by phosphorylating eIF2α and suppressing mTOR activity, thereby limiting the accumulation of ribosome collisions on damaged mRNA; Cells lacking GCN2 exhibit higher ribosome collision and excessive activation of RSR (ZAK), leading to sustained high activity of the JNK signaling pathway and higher rates of cell apoptosis; And the roles of ZAK and GCN2 in regulating cell response to UV damage are independent and do not affect each other.
After determining how ribosome collisions activate RSR, the authors focused on the attenuation of RSR signal transduction. The author analyzed the activation and degradation mechanisms of ZAK in response to RSR. Research has found that the activation of ZAK involves dynamic changes in multiple phosphorylation sites, and degradation is mainly mediated by the CRL1 E3 ubiquitin ligase complex. The self-phosphorylation sites of ZAK play an important role in degradation. The multi-domain structure of ZAK enables it to exert complex regulatory functions in response to ribosome collisions. Finally, the author investigated how the attenuation of RSR signal transduction affects the determination of cell fate. Through a series of experiments and analyses, the author found that ZAK degradation regulates JNK signaling through negative feedback, reduces cell apoptosis, and makes cells tolerant to sustained ribotoxic stress.
Overall, this article systematically studied various stress response activation modes after UV radiation-induced nucleic acid damage, revealing the important roles of various pathways in determining cell fate, especially how cell fate is driven by ribosome collision and ribotoxic stress. In-depth research on the activation and degradation mechanisms of ZAK can help develop new drugs targeting it to regulate cellular stress response, which has important potential for treating diseases related to stress response disorders.
Related Products and Services
Apoptosis Transcription Factors and Regulators Signal Transduction Proteins Cytokines Cancer Drug Targets Immune Checkpoint Proteins Protein Interaction Service Protein Expression and Purification Services Drug Discovery Screening Protein Pathway Profiling
Reference Sinha, Niladri & McKenney, Connor & Yeow, Zhong & Li, Jeffrey & Nam, Ki & Yaron-Barir, Tomer & Johnson, Jared & Huntsman, Emily & Cantley, Lewis & Ordureau, Alban & Regot, Sergi & Green, Rachel. (2024). The ribotoxic stress response drives UV-mediated cell death. Cell. 187. 10.1016/j.cell.2024.05.018.
