Uncategorized Friday, 2024/07/19
The use of adeno-associated virus (AAV) vectors for gene replacement is a promising method for treating many diseases. However, the packaging capacity of AAV (approximately 4.7 kilobases) poses a challenge to this therapeutic approach, limiting its application in diseases associated with larger protein-coding sequences, such as Duchenne muscular dystrophy where mRNA is 14 kilobases.
In a new study, researchers from the University of Washington have developed a novel gene therapy that utilizes the split intron-mediated protein trans-splicing mechanism to express larger dystrophin proteins, replacing defective DMD genes in muscles affected by Duchenne muscular dystrophy. They discovered several pairs of broken protein introns that can effectively connect two or three protein fragments, resulting in a medium-sized or full-length anti-muscle atrophy protein. The relevant research results were published online in the journal Nature, with the title "Split intein-mediated protein trans-splicing to express large dystrophins ".
Our Related Proteins
| Cat. No. | Product Name | Source | Species | Tag |
| DAG1-549H | Active Recombinant Human DAG1 Protein, His-tagged | Mammalian cells | Human | His |
| DAG1-1174R | Recombinant Rhesus monkey DAG1 Protein, His-tagged | Mammalian Cell | Rhesus Macaque | His |
| DAG1-620H | Recombinant Human DAG1 Protein, His-tagged | HEK293 | Human | His |
| DAG1-2327H | Recombinant Human DAG1 Protein, GST-tagged | Wheat Germ | Human | GST |
| SSPN-2858H | Recombinant Human SSPN Protein, MYC/DDK-tagged | HEK293 | Human | Myc&DDK |
| SSPN-301424H | Recombinant Human SSPN protein, GST-tagged | E.coli | Human | GST |
| SSPN-970HFL | Recombinant Full Length Human SSPN Protein, C-Flag-tagged | Mammalian cells | Human | Flag |
| SGCG-2629H | Recombinant Human SGCG, His-tagged | E.coli | Human | His |
| SGCA-3498H | Recombinant Human SGCA protein, His-tagged | E.coli | Human | His |
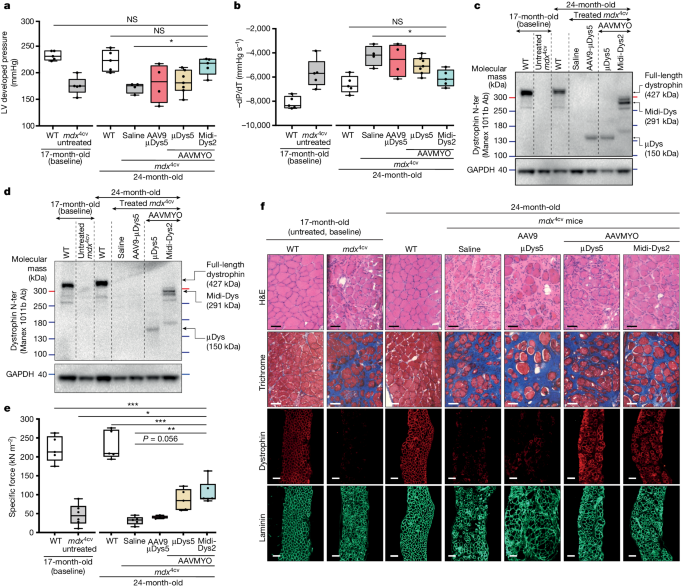
Their research suggests that delivering two or three types of AAV to mice with muscular dystrophy leads to robust expression of larger anti-atrophy proteins, resulting in significant improvement in their physiological condition. In addition, they demonstrated using the potent myotropic virus vector AAVMYO that a low total dose (2 × 10^13 viral genomes per kilogram) is sufficient to express large anti-muscle atrophy proteins in the striated muscles of the entire body and significantly improve the physiological condition of mice with muscular dystrophy. Their data shows that larger anti-muscle atrophy proteins have significant functional advantages compared to trace anti-muscle atrophy proteins currently being tested in clinical trials. Regardless of genotype, this method may benefit many patients with Duchenne muscular dystrophy or Becker muscular dystrophy, and may also be applicable to many other diseases caused by mutations in larger genes.
These research results indicate that the novel gene therapy developed by them for treating Duchenne muscular dystrophy not only has the potential to prevent muscle decline in patients with this genetic disease, but may also repair these damaged muscles in the future.
At present, there is no cure for Duchenne muscular dystrophy, and existing treatment methods and drugs can only slow down the condition. Patients with Duchenne muscular dystrophy are all male because this gene is located on the X chromosome. They begin to experience symptoms around the age of 4 and typically die in their twenties or thirties.
Professor Jeffery Chamberlain, the corresponding author of the paper and director of the Welston Muscular Atrophy Research Center at the University Of Washington School Of Medicine, has dedicated his entire career to finding treatments and seeking a cure for muscular atrophy.
Chamberlain pointed out that what has troubled scientists in the past is that the genes that need to be repaired are the largest genes in nature. So far, there is no way to fix enough protein in muscles. He said, "It's like giving a king-size bed, but you can't get it into your house
This new gene therapy has been successful in mouse models, using a series of adeno-associated viral vectors (AAV), which are tiny shuttles originating from viruses and are currently being used to deliver gene therapy to human cells. This gene therapy uses not one type of AAV, but a series of AAVs, each of which brings a portion of therapeutic anti-muscle atrophy protein into the muscle and embeds instructions, once inside the body, to begin assembling for necessary gene repair.
Returning to the metaphor of this big bed, not only are its parts shipped piece by piece, but the delivery workers also start assembling this big bed as soon as they enter the house. Chamberlain said that the next step for this gene therapy is human trials, which will begin in about two years.
In the laboratory, this gene therapy not only prevents the further development of the disease, but also reverses most of the pathological changes associated with malnutrition. Ultimately, Chamberlain and Hichem Tasfaout, as the first author of the paper, hope that this method can reverse muscle atrophy and restore normal muscle tissue health.
Chamberlain said that this new gene therapy also uses a novel AAV vector, AAVMYO, which allows for the use of lower doses, thus reducing or eliminating some of the side effects of previous methods.
Related Products and Services
Cytokines Cancer Drug Targets Immune Checkpoint Proteins Protein Engineering Services Protein Interaction Service Protein Expression and Purification Services Drug Discovery Screening Protein Pathway Profiling
Reference Hichem Tasfaout et al. Split intein-mediated protein trans-splicing to express large dystrophins. Nature, 2024, doi:10.1038/s41586-024-07710-8.
