Uncategorized Friday, 2024/11/15
Brown adipose tissue (BAT) plays an important role in mammalian energy metabolism, especially in cold environments, as it helps maintain body temperature through non-shivering thermogenesis. The thermogenesis process of BAT is activated by norepinephrine and relies on uncoupling protein 1 (UCP1) on the inner membrane of mitochondria. By consuming the electrochemical proton gradient within the mitochondria, energy is converted into heat, thereby achieving thermogenesis function. In addition, BAT can also help prevent cardiovascular metabolic diseases and certain types of cancer by consuming excess nutrients through heat production.
The thermogenic function of BAT is closely related to fatty acid oxidation (FAO), which supports mitochondrial uncoupling respiration by converting the energy generated by fatty acid oxidation into fuel for the TCA cycle. It is puzzling that while BAT is active in fatty acid oxidation, it also significantly increases fatty acid synthesis (FAS) in the cytoplasm, forming what is known as “future metabolic cycles”. This phenomenon appears to contradict its physiological function of energy consumption, especially considering that BAT is one of the most active tissues for lipid synthesis.
Acetyl CoA is a substrate for the synthesis of fatty acids and new lipids from non-lipid precursors such as glucose, while citrate lyase (ACLY) in the cytoplasm is the main catalytic enzyme for the production of acetyl CoA; Under specific conditions, member 2 of the acetyl CoA synthase short-chain family (ACSS2) can also generate acetyl CoA through acetic acid, providing another source for fatty acid synthesis. Under cold conditions, ACLY and ACSS2 expression are upregulated in BAT, accompanied by activation of glucose uptake and fat synthesis, but these synthesized fatty acids are not stored for a long time and are rapidly oxidized. Although the FAS-FAO cycle of fatty acid “synthesis oxidation” has been discovered for many years, its exact function and biological significance still need to be further studied. The energy metabolism mechanism of BAT is more complex than simple energy consumption on the surface.
Our Related Proteins
| Cat.No. # | Product Name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| ACLY-9291H | Recombinant Human ACLY protein, GST-tagged | E.coli | Human | GST | 751-1101 aa | |
| ACLY-2488H | Recombinant Human ACLY protein, His-tagged | Insect Cells | Human | His | Met1-Met1101 |
|
| ACLY-420H | Active Recombinant Human ACLY Protein, GST-tagged | Insect cells | Human | GST |
|
|
| UCP1-9176H | Recombinant Human UCP1, His-tagged | E.coli | Human | His | 96-185a.a. | |
| UCP1-27R | Recombinant Rat UCP1 protein, His-tagged | E.coli | Rat | His | 179-296 a.a. | |
| UCP1-28R | Recombinant Rat UCP1 protein, His/GST-tagged | E.coli | Rat | GST&His | 179-296 a.a. | |
| Ucp1-281M | Recombinant Mouse Ucp1 Protein, His-tagged | E.coli | Mouse | His | Pro179~Leu296 | |
| ACSS2-4019H | Recombinant Human ACSS2 protein, His-tagged | E.coli | Human | His | 18-191 aa | |
| ACSS2-209H | Recombinant Human ACSS2 Protein, GST-tagged | Wheat Germ | Human | GST |
|
|
| FASN-2661H | Recombinant Human FASN protein, His-tagged | E.coli | Human | His | 139 - 439 aa | |
| FASN-20H | Recombinant Human FASN, GST-tagged | Wheat Germ | Human | GST |
|
|
| FASN-138H | Recombinant Human FASN protein, MYC/DDK-tagged | HEK293 | Human | DDK&Myc |
|
|
| FASN-2275R | Recombinant Rat FASN Protein | Mammalian Cells | Rat | His |
|
|
| Fasn-596M | Recombinant Mouse Fasn protein, His/Myc-tagged | E.coli | Mouse | His&Myc | 2201-2504aa |
|
The David A. Guertin Laboratory at the University of Massachusetts Medical School led a research article titled “Brown fat ATP-citrate lyase links carbohydrate availability to thermogenesis and guards against metabolic stress” published in the journal Nature Metabolism, revealing the key role of ACLY in promoting thermogenesis by relieving metabolic stress in BAT. Research has found that the absence of ACLY in BAT can lead to TCA cycle overload, activate integrated stress response (ISR), and inhibit thermogenesis. In addition, knocking out the encoding genes of fatty acid synthase (Fasn) and Acly simultaneously in BAT can activate alternative metabolic pathways and restore normal thermogenesis function. This study provides new insights into the complex metabolic regulation relationship between the “synthesis oxidation” of fatty acids in BAT, which is contradictory.
In order to investigate the roles of ACLY and ACSS2 in BAT, the authors constructed transgenic mouse models (AclyBATKO and Acss2BATKO) with BAT tissue-specific knockout of Acly or Acss2. Using this model, the author found that the absence of ACLY leads to “whitening” of mouse BAT, increased tissue mass, and a rapid decrease in body temperature in cold environments. In contrast, the absence of ACSS2 did not cause similar physiological changes, indicating the critical role of ACLY in BAT thermogenesis and response to cold stimuli. In addition, the “whitening” of BAT in AclyBATKO mice indicates lipid accumulation, but the expression of lipid synthesis-related genes is actually reduced, suggesting that FAO function is inhibited at this time, rather than an increase in lipid synthesis, leading to the whitening of BAT. These changes are accompanied by the synthesis of new lipids, glucose uptake, Glut4 expression, and downregulation of lipid uptake-related genes, further indicating that ACLY deficiency in BAT inhibits FAO activity.
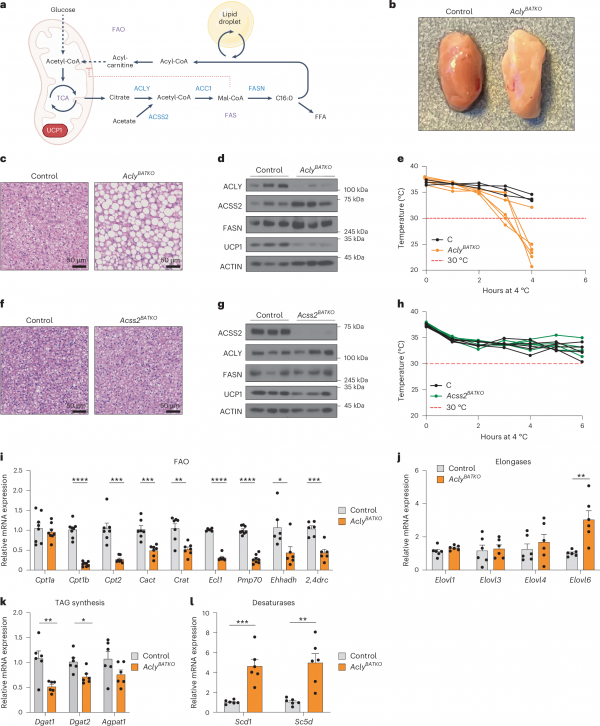
The study also found that the absence of Acly severely damaged mitochondrial function in BAT, manifested as a decrease in UCP1, ETC subunits, and various mitochondrial-related phospholipid levels, but no significant changes in mitochondrial ultrastructure were observed. In addition, the absence of ACLY in BAT significantly reduces the expression of key genes related to mitochondrial biogenesis and metabolism, leading to a decrease in thermogenesis and accompanied by a decrease in oxygen consumption rate and extracellular acidification rate. Therefore, ACLY plays a crucial role in maintaining mitochondrial function and uncoupling respiration in BAT, thereby regulating the thermogenic activity of BAT.
Long-term intense cold stress can induce brown-like adipocytes, known as beige or bright brown adipocytes, in subcutaneous white adipose tissue (WAT), a process called “browning” of WAT. In order to investigate whether ACLY is also crucial for the browning of WAT, the authors placed AclyFATKO adipose tissue-specific knockout mice (adipose tissue AclyFATKO) in a cold environment of 6 ° C for 4 weeks and found that mice lacking ACLY were unable to form beige/bright brown adipocytes in the cold environment, accompanied by decreased expression of UCP1, mitochondrial related genes, and thermogenic genes. Therefore, ACL not only regulates thermogenic function in BAT, but also affects the browning process of WAT under cold stress.
After further exploring the mechanism by which ACLY deficiency affects thermogenesis, the authors found that genes encoding mitochondria, respiratory chain, and lipid metabolism were downregulated in ACLY knockout-induced mice, while genes encoding inflammatory markers were upregulated. At the same time, ISR (Integrated Stress Response) and mitochondrial quality control pathways are also activated, thereby inhibiting thermogenesis and inducing metabolic remodeling. It can be seen that the absence of ACLY has a wide-ranging impact on the function of brown adipose tissue by triggering metabolic stress and ISR pathways. Next, the author conducted acyl CoA spectrum analysis and [13C] glucose isotope tracing experiments on AclyBATKO mice, and found that ACLY deficiency caused TCA cycle overload in brown adipose tissue, which in turn stimulated ISR, indicating that ACLY can alleviate metabolic stress and maintain normal BAT function by balancing the TCA cycle load.
It is worth noting that double knockout of Fasn and Acly can counteract BAT whitening and metabolic stress caused by ACLY deficiency. The double knockout of Fasn and Acly restored the levels of acetyl CoA and alleviated metabolic disorders caused by ACLY deficiency by regulating the carbon metabolism pathway. This indicates that the interaction between Fasn and ACLY is crucial for regulating the utilization of acetyl CoA and the balance of carbon flux. In addition, the double knockout of Fasn and Acly significantly inhibited the activation of the ISR signaling pathway and inflammatory response associated with metabolic stress. Therefore, the double knockout of Fasn and Acly in BAT rescued the metabolic imbalance caused by Acly deficiency by rebalancing the carbon flux and TCA cycle activity entering mitochondria during thermogenesis.
In summary, this study reveals the key role of ATP citrate lyase (ACLY) in alleviating metabolic stress and maintaining the thermogenic function of brown adipose tissue. The absence of ACLY can trigger TCA cycle overload and activate the integrated stress response, ultimately inhibiting thermogenesis. Knocking out Fasn and ACLY simultaneously can activate alternative metabolic pathways and restore normal thermogenesis. This study provides new insights into the complex relationship between fatty acid synthesis and oxidation in BAT.
Related Products and Services
Protein Expression and Purification Services
Reference
Korobkina ED, Calejman CM, Haley JA, Kelly ME, Li H, Gaughan M, Chen Q, Pepper HL, Ahmad H, Boucher A, Fluharty SM, Lin TY, Lotun A, Peura J, Trefely S, Green CR, Vo P, Semenkovich CF, Pitarresi JR, Spinelli JB, Aydemir O, Metallo CM, Lynes MD, Jang C, Snyder NW, Wellen KE, Guertin DA. Brown fat ATP-citrate lyase links carbohydrate availability to thermogenesis and guards against metabolic stress. Nat Metab. 2024 Oct 14. doi: 10.1038/s42255-024-01143-3. Epub ahead of print. PMID: 39402290.
