Uncategorized Tuesday, 2024/06/11
Obesity is a serious public health problem, which is related to a wide range of complications, including type 2 diabetes, cardiovascular disease, and liver steatosis. Dysfunction of lipid metabolism is a hallmark of obesity, related to the pathogenesis of these comorbidities. The imbalance between lipid synthesis and degradation leads to the accumulation of excess fat in adipose tissue and other organs.
Brown adipose tissue (BAT) or related beige adipose tissue is an induced form of adipocytes produced by subcutaneous white adipose tissue (WAT) under certain external stimuli. Therapeutic expansion or activation of brown adipose tissue (BAT) or related beige adipose tissue is a promising strategy for treating obesity and its complications.
Brown adipocytes and beige adipocytes have high mitochondrial content and mediate heat production through various mechanisms, most notably through a process involving uncoupling protein-1 (UCP1), a mitochondrial protein that separates respiration from ATP synthesis to generate heat. The activation of thermogenic fat can promote resting energy expenditure, systemic glucose processing, and insulin sensitivity.
Our Related Products
The liver is an important regulator of metabolic homeostasis. It is the center of lipid metabolism, coordinating lipid transport, synthesis, storage, and degradation in response to changes in nutrient availability and energy demand. Fatty acid oxidation is a process of fatty acid decomposition capacity and one of the main pathways of lipid degradation, in which the liver plays a crucial role.
Fatty acids can be oxidized in mitochondria and peroxisomes, where the first and rate-limiting steps of peroxisome beta oxidation are carried out by the acyl CoA oxidase protein family, which consists of three members: ACOX1, ACOX2, and ACOX3. Among them, ACOX1 is enriched in the liver. Acox1-/- severely damaged VLCFA β - oxidation in mouse liver.
Previous studies by the author have shown that mice with liver-specific knockout of ACOX1 exhibit normal performance and can prevent liver steatosis by inducing lipophagy (autophagic degradation of lipid droplets). Mechanistically speaking, acetyl CoA produced by acox1 mediated β - oxidation promotes the acetylation of Raptor, which is a component of the mTORC1 complex and can inhibit lipid synthesis. However, the impact of acox1 mediated β - oxidation in the liver on overall energy homeostasis, particularly in the context of diet-induced obesity, has not been studied.
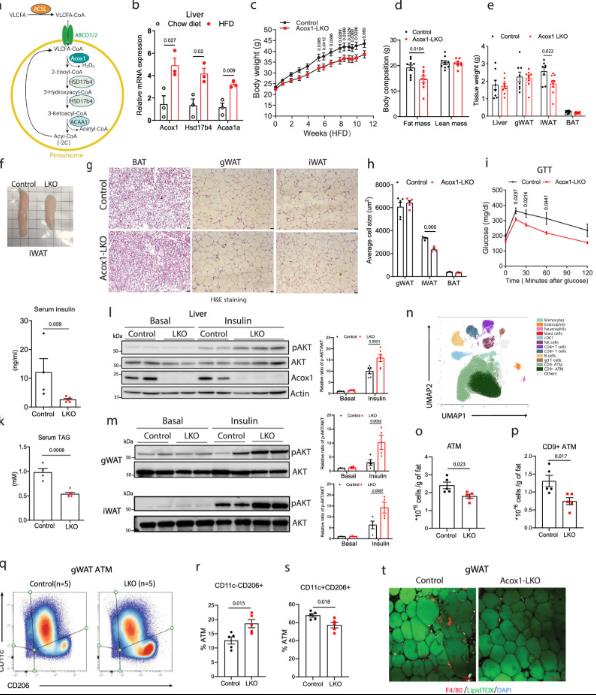
Recently, researchers from the Department of Endocrinology, Metabolism, and Lipids at the University of Washington School of Medicine published an article in the journal Nature Communications titled "Liver ACOX1 regulates levels of circulating lipids that promote metabolic health through adipose remodeling". The study found that liver peroxisome beta-oxidation is an important regulatory factor in metabolic homeostasis, which can affect energy expenditure and metabolic function of adipose tissue.
In the context of obesity, the content of β - oxidase acyl CoA oxidase 1 (ACOX1), which metabolizes long-chain fatty acids (VLCFA), increases, but it is still unclear how this pathway affects systemic energy metabolism.
In this study, researchers found that liver ACOX1-mediated β-oxidation regulation is involved in inter inter-organ communication of metabolic homeostasis. Liver-specific k-1-lko can protect mice from diet-induced obesity, adipose tissue inflammation, and systemic insulin resistance. In cultured white adipocytes, the serum of Acox1-L-K-O is considered the most important.
Global serum lipidomics shows that circulating levels of several omega 3 VLCFAs (C24-C28) increase, which have important physiological effects by activating mitochondria, promoting browning, mitochondrial biogenesis, and Glut4 translocation. This study identified that hepatic peroxisome beta-oxidation is an important regulatory factor for metabolic homeostasis, and demonstrated that by regulating ACOX1 or its metabolic substrates, we can effectively intervene in obesity-related metabolic disorders.
In summary, this study found that liver peroxisome β - oxidation is an important regulatory factor for metabolic homeostasis. By activating the GPR120 signal, it limits the circulating levels of endogenous omega-3 VLCFAs, thereby affecting the energy consumption and metabolic function of adipose tissue. Dietary supplementation of these beneficial lipids or inhibition of ACOX1 enzyme activity may be an effective strategy for treating obesity-related metabolic disorders.
Related Products and Services
CD Antigens Targets of CAR-T Cell Therapy Cytokines Cancer Drug Targets Immune Checkpoint Proteins Protein Interaction Service Protein Expression and Purification Services Drug Discovery Screening Protein Pathway Profiling
Reference Dongliang Lu et al. Liver ACOX1 regulates levels of circulating lipids that promote metabolic health through adipose remodeling. Nat Commun. 2024 May 17;15(1):4214. doi: 10.1038/s41467-024-48471-2.
