Uncategorized Tuesday, 2024/06/11
A new study published in the journal Science has brought us a new approach to the treatment of Alzheimer's disease (AD). Unlike many previous approaches to new drug development, this therapy does not target the key pathological proteins of Alzheimer's disease, beta-amyloid (Aβ) or tau protein, but rather targets the more fundamental neuronal calcium homeostasis.
Calcium ion disorders play an important pathological role in various types of dementia, including AD. In the preclinical stage of the disease, calcium ion disorders have persisted and directly led to synaptic loss, which is a key pathological event before neuronal death and memory impairment occur. A continuous increase in cytoplasmic calcium ion concentration was observed in both familial and idiopathic AD patients.
Of particular concern is the vicious cycle between calcium ion disorder and AD pathology. An increase in intracellular calcium ion concentration in diseased neurons can promote AD pathology, including the production of pathological Aβ and tau proteins. On the other hand, AD pathology can cause an increase in intracellular calcium ion concentration, trigger neurodegeneration, and ultimately lead to neuronal death.
Our Related Proteins
| Cat. No. | Product Name | Species | Source | Tag |
| MAPT-516H | Active Recombinant Human MAPT Protein | Human | E.coli | |
| MAPT-2874H | Recombinant Human MAPT, His-tagged | Human | E.coli | His |
| MAPT-528H | Recombinant Human MAPT protein(Ala2-Leu352), His-tagged | Human | E.coli | N-His |
| MAPT-1572H | Recombinant Human Microtubule-Associated Protein Tau | Human | E.coli | N/A |
| MAPT-121H | Recombinant Human Tau-441 (1-391) | Human | E.coli | N/A |
| APP-526H | Active Recombinant Human Amyloid Beta (A4) Precursor Protein, His-tagged | Human | Mammalian cells | His |
| APP-586H | Active Recombinant Human APP protein(Met1-Leu669), hFc-tagged | Human | HEK293 | C-hFc |
| APP-3850H | Recombinant Human APP, His & GST-tagged | Human | E.coli | His/GST |
| APP-1850H | Recombinant Human APP protein, GST-tagged | Human | E.coli | GST |
| APP-3646H | Recombinant Human APP protein, His-tagged | Human | E.coli | His |
| SEPT11-2582H | Recombinant Human SEPT11, GST-tagged | Human | E.coli | GST |
| SEPT6-357H | Recombinant Human SEPT6 Protein, His-tagged | Human | E.coli | His |
However, intervening in calcium ion signals is not an easy task, as calcium ion signals have an extremely wide impact on pathophysiology, making it difficult to precisely regulate abnormal calcium signals triggered by AD pathology.
To this end, researchers designed a cell-based drug screening method to stably express the frontal temporal dementia (FTD) related mutation P301L in human neuroblastoma, inducing tau protein hyperphosphorylation and aggregation, and leading to massive cell death. Previous studies have confirmed that in this model, cytotoxicity is associated with an increase in intracellular calcium ion concentration, and reducing intracellular calcium ion concentration can effectively reverse cell death.
Using this model to screen and optimize the compound library that regulates calcium ions through multiple rounds of structural optimization, researchers have identified a series of compounds with a common scaffold structure, named ReS19-T.
Using neurotoxic Aβ oligomers (ADDL) to treat rat neurons, ReS19-T (REM127 is one of them) can effectively restore neuronal dendritic spine density and avoid ADDL-induced neuronal death.
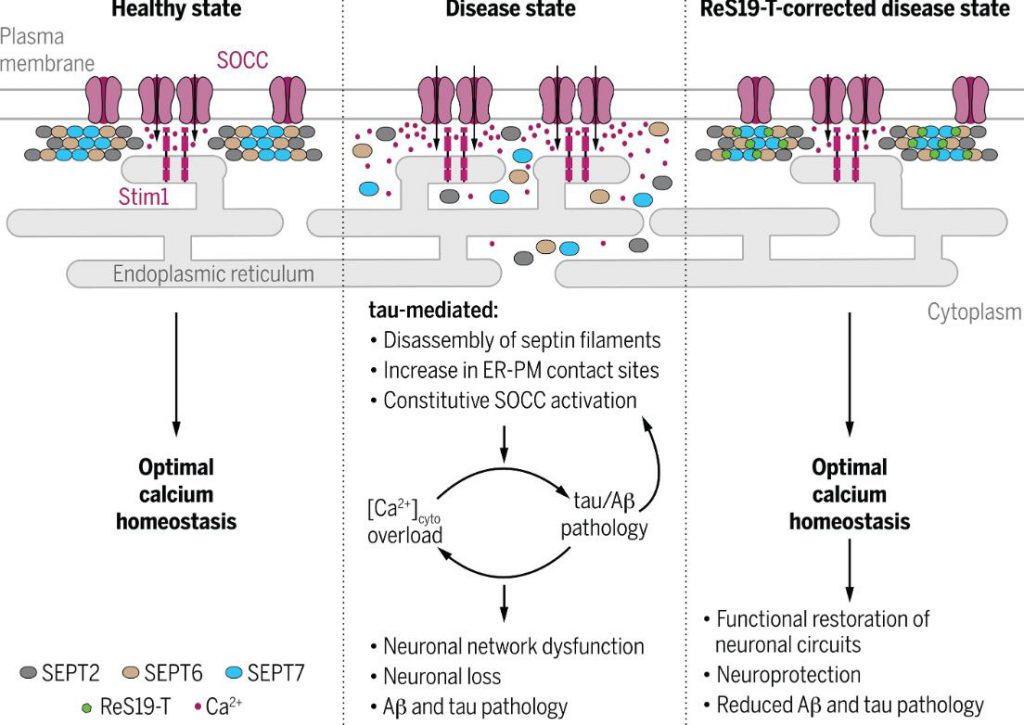
After identification, the main target of ReS19-T is septin6 (SEPT6), and it also has a weak interaction with SEPT11. Diaphragm proteins are a group of GTP-binding proteins that participate in a series of cellular physiological activities such as cytoskeleton and substance transport.
Diaphragm proteins are key regulatory factors for calcium storage channel manipulation (SOCC). Researchers have found that under disease conditions, pathological tau proteins can disrupt membrane protein assembly, and abnormally activate SOCC, leading to long-term increases in intracellular calcium levels and subsequent pathological physiological events.
ReS19-T acts like a glue, sticking together a loose membrane protein and restoring its normal function. There is no effect on SOCC under nonpathological conditions.
ReS19-T can effectively treat spatial memory deficits, and restore long-term hippocampal enhancement, and normal brain oscillatory activity in APP Ln mice.
After treating dual gene mutant AD mice with REM127 for 3 months, it was observed that the aggregation of Aβ plaques in the neocortex and hippocampus of the mice decreased by 55% -60%. AD-related microglial inflammation has also decreased.
In another type of AD model mouse, the APP-SAA mouse, which is mainly characterized by Aβ pathology, REM127 also achieved similar therapeutic effects.
In tauP301S mice, ReS19-T treatment significantly reduced the concentration of tau protein in the cerebrospinal fluid and the level of phosphorylated tau protein in the cortex.
It can be seen that ReS19-T can take effect on both major pathologies of AD.
It is expected that this mechanism does not only exist in AD. In various neurodegenerative diseases such as Parkinson's disease and FTD, expression disorders and localization errors of different subtypes of membrane proteins have been observed, indicating that regulating calcium homeostasis may become a general solution for the treatment of neurodegenerative diseases. The clinical trial of ReS19-T has been initiated since 2020 and has shown good safety in the Phase 1 trial. The specific results of the Phase 2a trial have not been announced yet. Let's look forward to the follow-up results.
Related Products and Services
Neurodegenerative Disease-related Proteins Alzheimer's Disease Targets of CAR-T Cell Therapy Cytokines Cancer Drug Targets Immune Checkpoint Proteins Protein Interaction Service Protein Expression and Purification Services Drug Discovery Screening Protein Pathway Profiling
Reference Princen K, Van Dooren T, van Gorsel M, et al. Pharmacological modulation of septins restores calcium homeostasis and is neuroprotective in models of Alzheimer's disease. Science. 2024 May 31;384(6699):eadd6260. doi: 10.1126/science.add6260. Epub 2024 May 31. PMID: 38815015.
