Uncategorized Friday, 2024/11/15
Cancer cells excrete metabolic byproducts such as lactate into the surrounding tumor microenvironment during their growth process. Recently, researchers at the University of Pittsburgh found that these lactic acids can cause T cells (an important immune cell) to lose their anti-cancer ability. When T cells run out of energy and become functionally depleted, they take up these lactic acids, further weakening their combat effectiveness. However, when researchers blocked the protein that inputs lactate into cells, these exhausted T cells surprisingly regained their vitality, improving tumor control in mouse cancer models.
The relevant research results were published online in the journal Nature Immunology, with the title “Dysfunction of exhausted T cells is enforced by MCT11-mediated lactate metabolism”.
When T cells are continuously exposed to the tumor environment, they gradually lose their effectiveness. This is because the surface of T cells expresses some co-inhibitory receptors like breaks, such as PD-1 and CTLA-4. These receptors gradually cause T cells to lose their ability to kill cancer and eventually enter a state of terminal failure. Currently, most immunotherapies, such as anti-PD-1 and anti-CTLA-4 drugs, restore T cell function by blocking these co-inhibitory receptors. However, these therapies have not achieved the expected results in many cancers.
Ours Related Proteins
| Cat.No. # | Product Name | Source (Host) | Species | Tag | Protein Length | Price |
|---|---|---|---|---|---|---|
| SLC16A11-15229M | Recombinant Mouse SLC16A11 Protein | Mammalian Cells | Mouse | His |
|
|
| SLC16A11-1801HCL | Recombinant Human SLC16A11 293 Cell Lysate | HEK293 | Human | Non |
|
|
| PDCD1-172H |
Active Recombinant Human PDCD1, no tag
|
HEK293 | Human | Non | Leu 25 - Leu 288 | |
| PDCD1-1222RAF488 |
Active Recombinant Monkey PDCD1 Protein, His-tagged, Alexa Fluor 488 conjugated
|
HEK293 | Monkey | His | 178 | |
| PDCD1-031HAF488 |
Active Recombinant Human PDCD1 Protein, MIgG2a mFc-tagged, Alexa Fluor 488 conjugated
|
CHO | Human | mFc | 25-167 a.a. | |
| PDCD1-032HAF488 |
Active Recombinant Human PDCD1 Protein, hFc-tagged, Alexa Fluor 488 conjugated
|
CHO | Human | Fc | 25-167 a.a. | |
| CTLA4-109CAF488 |
Active Recombinant Cynomolgus CTLA4 Protein, His-tagged, Alexa Fluor 488 conjugated
|
HEK293 | Monkey | His | Met1-Asp161, 136 |
In order to find a new way to activate T cells with functional failure, Delgoffe and his team studied a protein family called solute carrier (SLC). These proteins are responsible for delivering nutrients to cells. They found that the expression of a solute carrier called MCT11 (responsible for inputting lactate) was significantly increased in terminally depleted T cells. This suggests that lactate may be the main culprit leading to the loss of T cell function.
When researchers removed the gene encoding MCT11 in mice or blocked the protein with monoclonal antibodies, the lactate intake by T cells decreased. As a result, T cells showed better function and tumor control ability in mouse models of melanoma, colorectal cancer, and head and neck cancer.
Delgoffe explained, “If we compare the co-inhibitory receptors that cause T cell failure to the brakes of a car, then lactate is like poor quality gasoline contaminated with dirt and particles, hindering the performance of the car. By blocking access to gas stations that sell this poor-quality fuel, cars can obtain better gasoline, thereby improving performance. Similarly, by blocking MCT11, we can prevent T cells from obtaining lactate that hinders their function.”
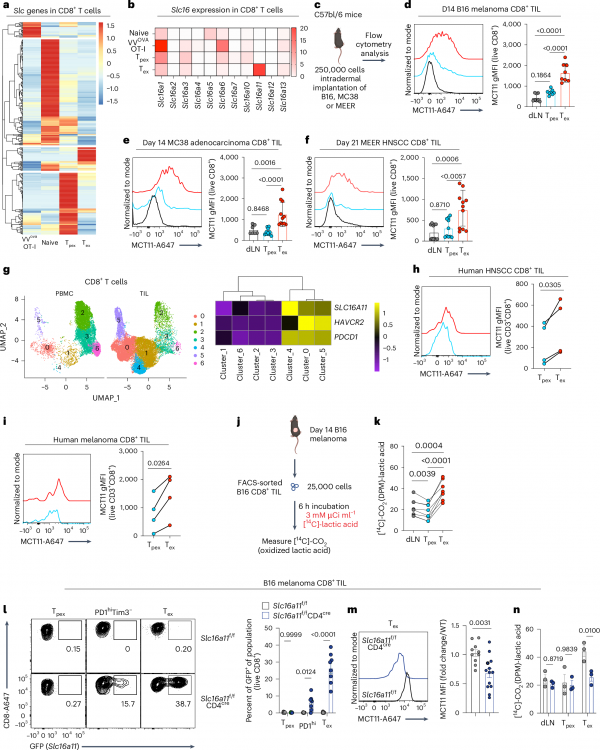
The Delgoffe team also found that using the MCT11 antibody alone can promote tumor clearance in mice, but the effect is better when combined with anti-PD-1. Delgoffe and the first author of the paper, Dr. Ronal Peralta, a postdoctoral fellow in the Delgoffe laboratory, are currently optimizing the effectiveness of MCT antibodies in human T cells through their newly established independent company, with the goal of testing them in future clinical trials.
Peralta stated that MCT11 is an attractive therapeutic target because it is almost exclusively expressed in exhausted T cells, which are concentrated in tumors. This means that drugs targeting MCT11 have fewer side effects compared to traditional immunotherapy that acts on T cells throughout the body, such as anti-PD-1.
Peralta said, “This study is indeed exciting because it conceptually validates how targeted T cells interact with metabolites in their environment to promote better outcomes in cancer treatment. This opens the door for us to explore how to use other targets in immune cells to treat cancer and many other diseases.”
This study not only reveals the role of lactate in T cell failure, but also provides a new strategy to reactivate dysfunctional T cells by blocking lactate input. This brings new hope for future cancer treatment, which may lead to better treatment outcomes for patients who have not responded to traditional immunotherapy.
Related Products and Services
Protein Expression and Purification Services
Reference
Ronal M. Peralta et al. Dysfunction of exhausted T cells is enforced by MCT11-mediated lactate metabolism. Nature Immunology, 2024, doi:10.1038/s41590-024-01999-3.
